Abstract
Background: Autologous T cells engineered to express a CD19-directed chimeric antigen receptor (CAR) have shown high overall response rates in treatment-refractory CD19+ B-cell non-Hodgkin lymphoma (NHL). However, over half of these patients will have lymphoma that fails to achieve remission or will relapse; thus, strategies to further improve the long-term efficacy of CAR-T cell products are needed. NKTR-255 is a novel investigational polymer-conjugated IL-15 agonist, designed to activate, proliferate and expand natural killer (NK) and CD8+ T-cells in vivo. NKTR-255 also promotes the survival and expansion of memory CD8+ T cells. Preclinical data in B-cell lymphoma xenograft models have shown that NKTR-255 enhanced expansion, survival, and anti-tumor activity of human CD19 CAR-T cells. Further, clinical studies have demonstrated that NKTR-255 promoted CD8+ T cell expansion in patients with relapsed/refractory (r/r) NHL who previously received CAR-T cell therapy (NCT04136756). Here, we describe a planned Phase 2/3 clinical trial of NKTR-255 following CAR-T cell therapy.
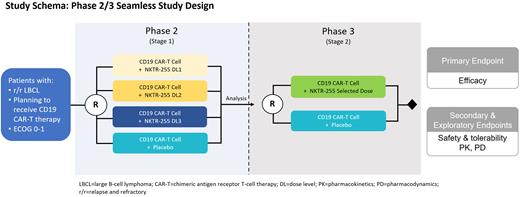
Methods: This is a phase 2/3, randomized, double blind, placebo-controlled, multicenter study of NKTR-255 vs placebo following CD-19 directed CAR-T therapy. Eligible patients who have r/r large B-cell lymphoma and are planned for treatment with an FDA-approved CAR-T product (axi-cel or liso-cel) will be enrolled. All patients will receive initial study drug administration of NKTR-255 intravenously, starting approximately 14 days following CAR-T therapy, with continued dosing every 21 days. The study will be comprised of two stages: 1) Phase 2: patients will be randomized to placebo or one of multiple dose cohorts of NKTR-255 (Stage 1) and 2) Phase 3: patients will be randomized to placebo or the selected NKTR-255 dose regimen (Stage 2). The primary objective for Stage 1 is to identify the dose of NKTR-255 for the Phase 3 part of the study based on the safety and tolerability of NKTR-255 as well as the complete response rate (CRR) at month 6. The primary efficacy endpoints of Stage 2 are CRR at month 6 and event-free survival. Efficacy analyses will be performed separately for Stage 1 and Stage 2. Efficacy, as measured by PET-CT, will be evaluated according to the Lugano Criteria (Cheson 2014). The key eligibility criteria following CD19 targeted CAR-T cell infusion include no grade ≥ 1 cytokine release syndrome (CRS) on the day of NKTR-255 administration, no grade ≥ 3 CRS within 72 hours proceeding NKTR-255 administration, no grade ≥ 3 immune effector cell-associated neurotoxicity (ICANS) of > 72 hours duration, no grade ≥ 2 ICANS on the day of NKTR-255 infusion and no tocilizumab and/or dexamethasone within 48 hours proceeding NKTR-255 administration.
Conclusions: Based on preclinical and clinical evidence, NKTR-255 has the potential to improve efficacy of currently approved cellular therapies. This trial evaluates safety and efficacy of NKTR-255 following commercial CD19 CAR-T therapy to enhance antitumor effect and durability of responses.
Disclosures
Perales:Takeda: Honoraria; Karyopharm: Honoraria; MorphoSys: Consultancy, Honoraria; VectivBio AG: Honoraria; Bellicum: Honoraria; Medigene: Consultancy; Servier: Consultancy; DSMB: Other; Sellas Life Sciences: Consultancy; Cidara Therapeutics: Consultancy; Celgene: Honoraria; Vor Biopharma: Honoraria; Omeros: Consultancy; Nektar Therapeutics: Consultancy, Honoraria; Orca Bio: Consultancy; Astellas: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Miltenyi Biotec: Consultancy, Honoraria; Merck: Consultancy; Kite, a Gilead Company: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Bristol-Mysers Squibb: Honoraria. Ahmed:Myeloid Therapeutics: Consultancy; Tessa Therapeutics: Consultancy, Research Funding; Xencor: Research Funding; Chimagen: Consultancy, Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Seagen: Research Funding; Merck: Research Funding. Riedell:Karyopharm: Membership on an entity's Board of Directors or advisory committees; Calibr: Research Funding; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Research Funding; Tessa Therapeutics: Research Funding; MorphoSys: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sana Biotechnology: Consultancy; Janssen: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Research Funding; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. McGuirk:BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Nextar: Consultancy, Honoraria; Orca Bio: Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Sana: Honoraria; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial. Oluwole:ADC Therapeutics: Consultancy; Kite, a Gilead Company: Research Funding; Janssen: Consultancy; Curio Science: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; TG Therapeutics: Consultancy. Chaudhry:Nektar Therapeutics: Current Employment. Lee:Nektar Therapeutics: Current Employment. Dai:Nektar Therapeutics: Current Employment. Dixit:Nektar Therapeutics: Current Employment. Fanton:Nektar Therapeutics: Current Employment. Marcondes:Nektar Therapeutics: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Zalevsky:Nektar Therapeutics: Current Employment. Tagliaferri:Nektar Therapeutics: Current Employment. Turtle:Juno Therapeutics, a BMS Company: Patents & Royalties, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Decheng Capital: Consultancy, Membership on an entity's Board of Directors or advisory committees; Expert Connect: Consultancy; Allogene: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Caribou Bioscience: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Eureka Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; T-CURX: Membership on an entity's Board of Directors or advisory committees; Kite Pharma, a Gilead Company: Membership on an entity's Board of Directors or advisory committees; Century Therapeutics: Membership on an entity's Board of Directors or advisory committees; Arsenal Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Precision Bioscience: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prescient Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal